Describe a Phospholipid Molecule and Its Interaction With Water
The non-polar hydrophobic water-repelling tails are sandwiched between the heads and are protected from the aqueous environments. The BCR is composed of surface-bound IgD or IgM antibodies and associated Ig-α and Ig-β heterodimers.
Phospholipids Introduction To Chemistry
The polar hydrophilic water-loving heads face the outside and inside of the cell.
. It is part of the B cell receptor BCR which allows a B cell to detect when a specific antigen is present in the body and triggers B cell activation. A positive charge typically aligns the water dipole lined up with the bisector of the two OH bonds within the water molecule as has previously been shown for water hydrating ions 162. A phospholipid has both hydrophobic and hydrophilic regions.
The membrane-bound form of an antibody may be called a surface immunoglobulin sIg or a membrane immunoglobulin mIg. The fatty acid chains are hydrophobic and exclude themselves from water whereas the phosphate is hydrophilic and interacts with water. Cells are surrounded by a membrane which has a bilayer of phospholipids.
The fatty acids of phospholipids face inside away from water whereas the phosphate group. These heads interact with the aqueous environment outside and within a cell. Scientists Singer and Nicolson described the structure of the phospholipid.
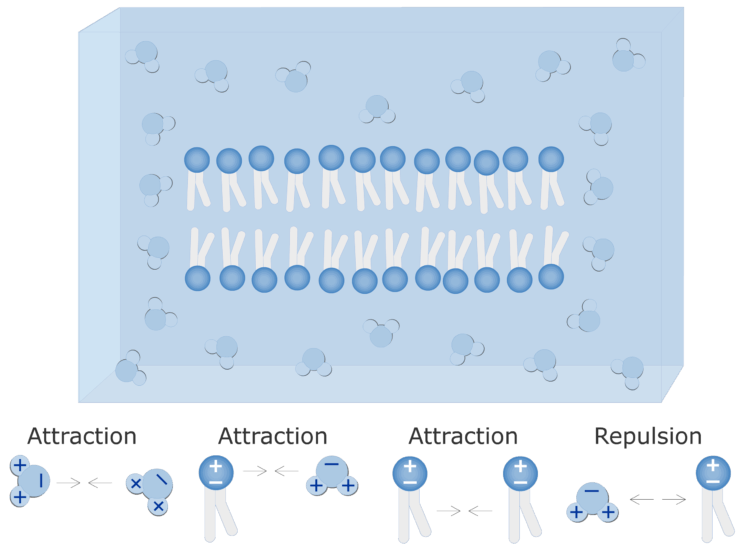
Water Cell Membrane Interactions


Comments
Post a Comment